The U.S. Food and Drug Administration on Tuesday issued a “do not use” warning about the Skippack Medical Lab SARS-CoV-2 Antigen Rapid Test (Colloidal Gold) because it “is not authorized, cleared, or approved by the FDA for distribution or use in the United States.”
The FDA said it is concerned about the risk of receiving false results because the company, SML Distribution LLC, “has not provided the FDA with adequate data to show the test works correctly.”
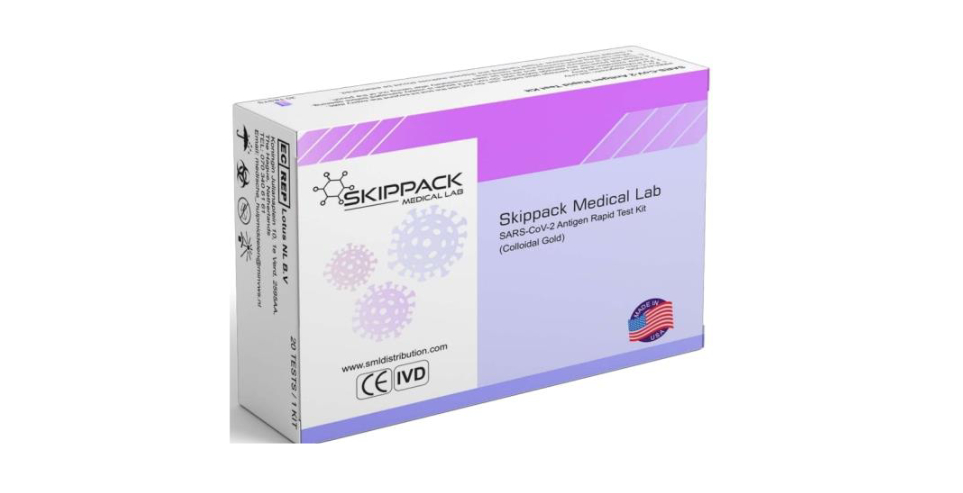
SML Distribution, the FDA said, has recalled the tests.
The test uses a nasal swab to detect the proteins from the virus that causes COVID-19.
SML Distribution, based in Tyrone, Pa., recalled up to 209,450 of the tests on March 4. They were distributed in the United States from Jan. 21, 2022, to Feb. 11, 2022.
For more information, call SML Distribution at 888-209-4406 or send an email to techsupport@smldistribution.com.
The FDA has previously issued warnings about other COVID-19 tests it has not authorized.